
The surface tension of water is `75(dyne)/(cm)`. Find the minimum vertical force required to pull a - YouTube

Using data from the diagram below, calculate the ratio of the height to which water at 0 C and mercury are raised or suppressed by capillary action in the same glass tube. (

Calculated surface tension of water-oil with respect to temperature.... | Download Scientific Diagram

11.8 Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action – College Physics chapters 1-17
![Calculate the height to which water will rise in a capillary tube of diameter 1xx10^(-3)m [given surface tension of water is 0.072Nm^(-1) angle of contact is 0^(@),g=9.8ms^(-2) and density of water =1000kgm^(-3)] Calculate the height to which water will rise in a capillary tube of diameter 1xx10^(-3)m [given surface tension of water is 0.072Nm^(-1) angle of contact is 0^(@),g=9.8ms^(-2) and density of water =1000kgm^(-3)]](https://d10lpgp6xz60nq.cloudfront.net/ss/web/365220.jpg)
Calculate the height to which water will rise in a capillary tube of diameter 1xx10^(-3)m [given surface tension of water is 0.072Nm^(-1) angle of contact is 0^(@),g=9.8ms^(-2) and density of water =1000kgm^(-3)]

Calculate the rise of water inside a clean glass capillary tube of radius 0.1 mm , when immersed in water of surface tension 7 × 10^-2 N/m . The angle of contact

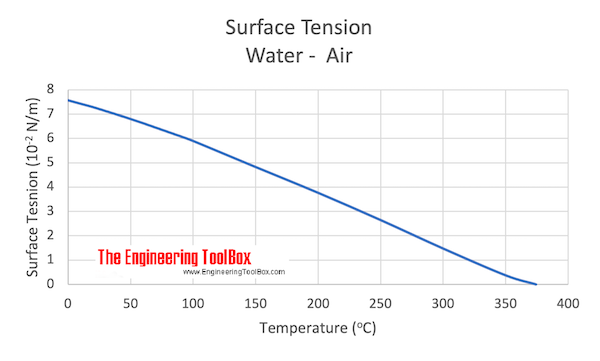
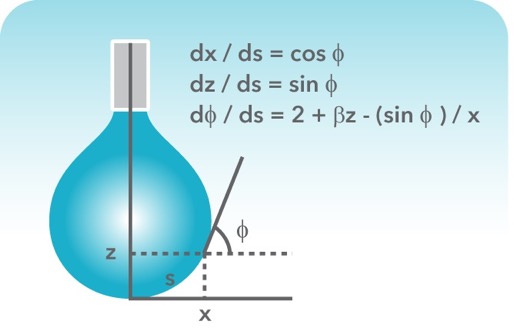



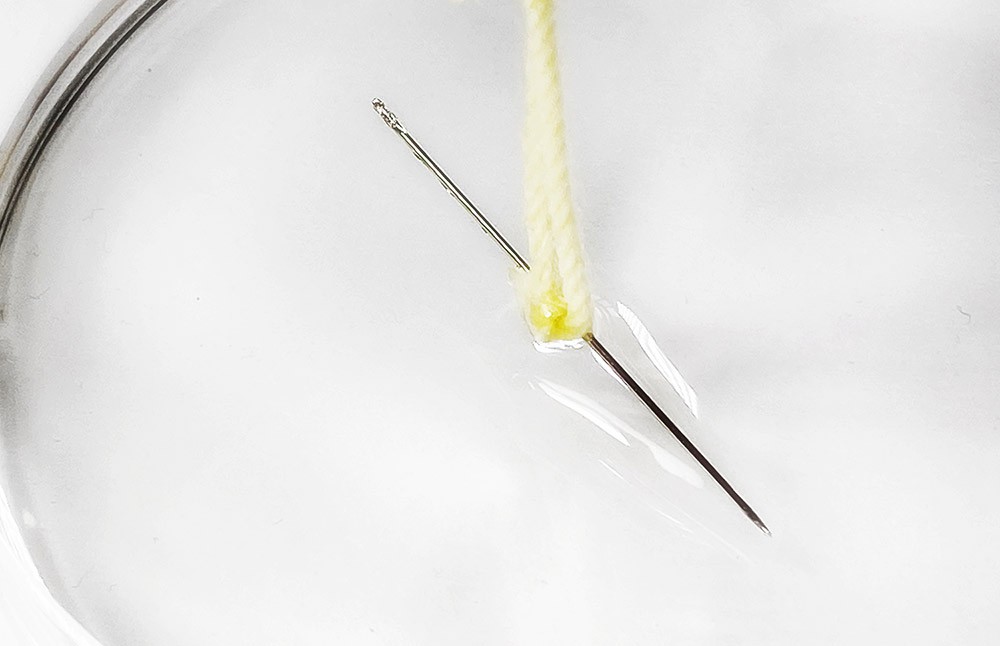




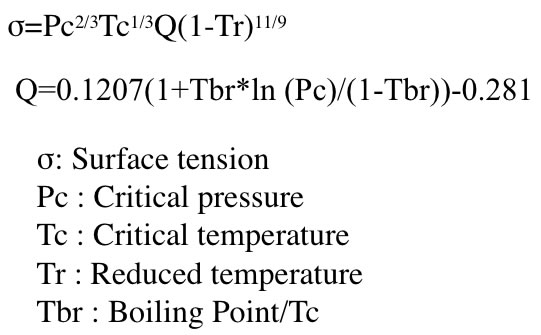

![Values of the surface tension of water at different temperatures [1]. | Download Table Values of the surface tension of water at different temperatures [1]. | Download Table](https://www.researchgate.net/profile/Concetto-Gianino/publication/231143777/figure/tbl1/AS:668494844686342@1536392999595/Values-of-the-surface-tension-of-water-at-different-temperatures-1_Q320.jpg)

